AEMD) Declares Monetary Outcomes for the Fiscal Third Quarter Ended December 31, 2024 and Offers Company Replace
SAN DIEGO – February 12, 2025 (Investorideas.com Newswire) Aethlon Medical, Inc. (Nasdaq: AEMD), a medical therapeutic firm centered on growing merchandise to deal with most cancers and life-threatening infectious illnesses, at present reported monetary outcomes for its fiscal third quarter ended December 31, 2024 and supplied an replace on current developments.
Paid Information Dissemination of behalf of AEMD.
Firm Updates
Through the third quarter, and subsequently, the corporate made important progress in its oncology trial efforts in Australia whereas executing cost-cutting measures to reinforce operational effectivity. Administration is happy to spotlight the next key developments:
Medical Trials:
Regular progress in our Australian Oncology trial of the Hemopurifier in sufferers with stable tumors was made. To this point, three sufferers have been enrolled. Two sufferers didn’t advance to the therapy part as a consequence of pre-specified stopping standards through the run-in interval – one confirmed a medical response to anti PD-1 remedy, whereas the opposite skilled toxicity associated to anti-PD-1 remedy. The third affected person, who didn’t reply to anti-PD-1 remedy, efficiently underwent a single 4-hour Hemopurifier therapy at Royal Adelaide Hospital on January 29, 2025. The therapy was accomplished with no device-related points or problems. Samples collected earlier than and after therapy can be analyzed to evaluate extracellular vesicle elimination and modifications in anti-tumor T cell exercise. This knowledge can be out there as soon as all 3 sufferers on this affected person cohort are handled.
Following the investigator assembly with the three medical websites, Aethlon obtained helpful suggestions suggesting protocol modifications that would presumably enhance enrollment pace, cut back display screen failures, and shorten the time to Hemopurifier therapy and time to knowledge. In response, the Aethlon workforce swiftly developed a protocol modification incorporating these suggestions.
Key modifications embody enrolling sufferers solely after they’ve been confirmed to not be responding to anti-PD-1 remedy. This adjustment eliminates the necessity to establish sufferers inside the first 2 weeks of beginning anti-PD-1 remedy and removes the two-month run-in interval beforehand required to evaluate response to remedy. Moreover, restrictions on generally prescribed concomitant drugs that don’t affect affected person security have been lifted. The amended protocol additionally broadens eligibility to incorporate sufferers receiving all authorized dosing regimens of Pembrolizumab and Nivolumab, quite than limiting enrollment to particular schedules.
The corporate is happy to announce that the Human Analysis Ethics Committees (HREC) and Analysis Governance Places of work (RGO) have authorized this modification in any respect three medical websites. The 2 at present lively medical websites, Royal Adelaide Hospital and Pindara Personal Hospital, can enroll beneath the amended protocol. The third web site, Genesis Care/ Royal North Shore Hospital, can start enrollment beneath this modification following a Web site Initiation Go to (SIV) on February 14, 2025.
The corporate continues to pursue approval of the same medical trial in India. HREC approval has been obtained at Medanta Medicity Hospital, and we’re at present awaiting approval from the regulatory company CDSCO in India. Latest regulatory modifications in India have launched extra documentation necessities that have been beforehand not vital. Aethlon is actively responding to CDSCO’s queries by way of the corporate’s India CRO, Qualtran.
Operational Effectivity:
Aethlon has applied strategic cost-cutting measures to optimize firm sources, enabling it to keep up a robust give attention to the high-impact oncology trials in each Australia and India. These initiatives are designed to enhance useful resource allocation, cut back operational bills, and assist the continued development of our medical packages.
“Through the third fiscal quarter and subsequent interval, we continued to advance our oncology trials, together with therapy of the primary affected person at Royal Adelaide Hospital in late January. We’re happy to report that the affected person tolerated the process with out problems, making a essential milestone for the protection, feasibility and dose-finding trials of the Hemopurifier in sufferers with stable tumors who haven’t responded to anti-PD-1 antibodies,” acknowledged James Frakes, Chief Government Officer and Chief Monetary Officer of Aethlon Medical. “We at present have two medical websites activated and open for enrollment in Australia, with a 3rd web site anticipated to be activated in February 2025. As well as, we have now obtained ethics committee approval from a web site in India. We additionally anticipate continued enrollments in our Hemopurifier most cancers trial as these websites progress.
Operational Effectivity:
Aethlon has applied strategic cost-cutting measures to optimize firm sources, enabling it to keep up a robust give attention to the high-impact oncology trials in each Australia and India. These initiatives are designed to enhance useful resource allocation, cut back operational bills, and assist the continued development of our medical packages.
“Through the third fiscal quarter and subsequent interval, we continued to advance our oncology trials, together with therapy of the primary affected person at Royal Adelaide Hospital in late January. We’re happy to report that the affected person tolerated the process with out problems, making a essential milestone for the protection, feasibility and dose-finding trials of the Hemopurifier in sufferers with stable tumors who haven’t responded to anti-PD-1 antibodies,” acknowledged James Frakes, Chief Government Officer and Chief Monetary Officer of Aethlon Medical. “We at present have two medical websites activated and open for enrollment in Australia, with a 3rd web site anticipated to be activated in February 2025. As well as, we have now obtained ethics committee approval from a web site in India. We additionally anticipate continued enrollments in our Hemopurifier most cancers trial as these websites progress.
At the moment, solely roughly 30% of sufferers who obtain pembrolizumab or nivolumab could have lasting medical responses to those brokers. Extracellular vesicles (EVs) produced by tumors have been implicated within the unfold of cancers in addition to the resistance to anti-PD-1 therapies. The Aethlon Hemopurifier has been designed to bind and take away these EVs from the bloodstream, which can enhance therapeutic response charges to anti-PD-1 antibodies. In preclinical research, the Hemopurifier has been proven to scale back the variety of EVs in most cancers affected person plasma samples.
The corporate is intently monitoring developments associated to Fowl Flu in the US, Marburg virus in Rwanda and Ebola virus in Uganda. Aethlon has direct expertise with these viruses, having beforehand generated in vitro viral binding knowledge for all three viruses and handled an Ebola affected person in Germany beneath Emergency Use situations. Aethlon will proceed to watch these conditions rigorously and be poised to reply if at present out there therapy methods are deemed ineffective.
Monetary Outcomes for the Fiscal Third Quarter Ended December 31, 2024
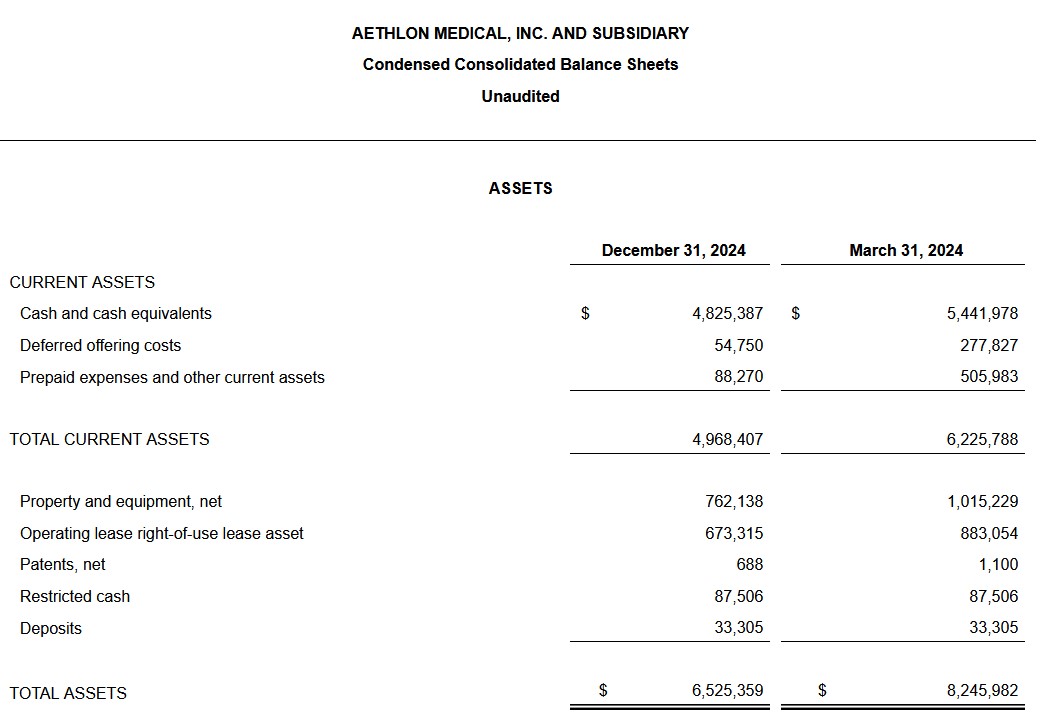
As of December 31, 2024, Aethlon had a money stability of roughly $4.8 million.
Consolidated working bills for the fiscal quarter ended December 31, 2024, decreased by roughly $1.8 million, or roughly 50%, to $1.8 million in comparison with $3.6 million for the fiscal quarter ended December 31, 2023. This discount was pushed by a $1.3 million lower in payroll and associated bills, a $300,000 lower in skilled charges, and a $200,000 lower usually and administrative bills.
The approximate $1.3 million lower in payroll and associated bills was primarily attributable to a discount of $900,000 in separation bills associated to the Separation Settlement with the previous Chief Government Officer that had been recorded within the December 2023 interval, in addition to a lower of roughly $400,000 as a consequence of a discount in headcount. Of the approximate $900,000 of separation bills associated to the departure of the previous CEO, roughly $400,000 associated to the acceleration of vesting of inventory choices.
The approximate $300,000 lower in skilled charges was primarily as a consequence of an approximate discount of $200,000 in authorized charges ensuing from the transition to a brand new authorized agency, and a lower of $200,000 in scientific and operational consulting charges largely attributable to accomplished initiatives. These decreases have been partially offset by an approximate $100,000 improve in investor relations and accounting charges.
The approximate $200,000 lower usually and administrative bills was primarily pushed by a $300,000 discount in provides, largely associated to the uncooked supplies and elements used within the manufacturing of the Hemopurifier, with no comparable purchases through the present interval. Moreover, there was an approximate $100,000 lower in insurance coverage bills related to a diminished headcount and varied different working bills. These reductions have been partially offset by a $200,000 improve in bills associated medical trial bills associated to our ongoing oncology medical trials in Australia and India.
Because of the components famous above, the corporate’s internet loss decreased to roughly $1.8 million within the fiscal quarter ended December 31, 2024, from roughly $3.5 million within the fiscal quarter ended December 31, 2023.
The consolidated stability sheet for December 31, 2024, and the consolidated statements of operations for the three- and nine-month intervals ended December 31, 2024 and 2023 observe on the finish of this launch.
Convention Name
Administration will host a convention name at present, Wednesday, February 12, 2025, at 4:30 p.m. ET to evaluation the corporate’s monetary outcomes and up to date company developments. Following administration’s formal remarks, there can be a query and reply session.
events can register for the convention name by navigating to https://dpregister.com/sreg/10196811/fe7c419c9d. Please be aware that registered individuals will obtain their dial-in quantity upon registration.
events with out web entry or unable to pre-register might dial in by calling:
PARTICIPANT DIAL IN (TOLL FREE): 1-844-836-8741
PARTICIPANT INTERNATIONAL DIAL IN: 1-412-317-5442
All callers ought to ask for the Aethlon Medical, Inc. convention name.
A replay of the decision can be out there roughly one hour after the tip of the decision by way of March 12, 2025. The replay will be accessed through Aethlon Medical’s web site or by dialing 1-877-344-7529 (home) or 1-412-317-0088 (worldwide) or Canada toll free at 1-855-669-9658. The replay convention ID quantity is 7828175.
About Aethlon and the Hemopurifier®
Aethlon Medical is a medical therapeutic firm centered on growing the Hemopurifier, a medical stage immunotherapeutic gadget which is designed to fight most cancers and life-threatening viral infections and to be used in organ transplantation. In human research, the Hemopurifier has demonstrated the elimination of life-threatening viruses and in pre-clinical research, the Hemopurifier has demonstrated the elimination of dangerous exosomes from organic fluids, using its proprietary lectin-based know-how. This motion has potential functions in most cancers, the place exosomes might promote immune suppression and metastasis, and in life-threatening infectious illnesses. The Hemopurifier is a U.S. Meals and Drug Administration (FDA) designated Breakthrough Gadget indicated for the therapy of people with superior or metastatic most cancers who’re both unresponsive to or illiberal of normal of care remedy, and with most cancers sorts through which exosomes have been proven to take part within the improvement or severity of the illness. The Hemopurifier additionally holds an FDA Breakthrough Gadget designation and an open Investigational Gadget Exemption (IDE) software associated to the therapy of life-threatening viruses that aren’t addressed with authorized therapies.
Extra data will be discovered at www.AethlonMedical.com.
Ahead-Trying Statements
This press launch comprises forward-looking statements inside the which means of Part 27A of the Securities Act of 1933 and Part 21E of the Securities Trade Act of 1934 that contain dangers and uncertainties. Statements containing phrases resembling “might,” “imagine,” “anticipate,” “count on,” “intend,” “plan,” “mission,” “will,” “projections,” “estimate,” “probably” or comparable expressions represent forward-looking statements. Such forward-looking statements are topic to important dangers and uncertainties and precise outcomes might differ materially from the outcomes anticipated within the forward-looking statements. These forward-looking statements are primarily based upon Aethlon’s present expectations and contain assumptions that will by no means materialize or might show to be incorrect. Elements that will contribute to such variations embody, with out limitation, the Firm’s capacity to boost extra capital on phrases favorable to the Firm, or in any respect; the Firm’s capacity to efficiently full improvement of the Hemopurifier; the Firm’s capacity to efficiently display the utility and security of the Hemopurifier in most cancers and infectious illnesses and within the transplant setting; the Firm’s capacity to attain and notice the anticipated advantages from potential milestones; the Firm’s capacity to acquire approval from the Ethics Committee of its third location in Australia, together with on the timeline anticipated by the Firm; the Firm’s capacity to enroll extra sufferers in its oncology medical trials in Australia and India, together with on the timeline anticipated by the Firm; the Firm’s capacity to handle and efficiently full its medical trials; the Firm’s capacity to efficiently manufacture the Hemopurifier in enough portions for its medical trials; unexpected modifications in regulatory necessities; the Firm’s capacity to remedy deficiencies and proceed to keep up its Nasdaq itemizing; and different potential dangers. The foregoing checklist of dangers and uncertainties is illustrative, however shouldn’t be exhaustive. Extra components that would trigger outcomes to vary materially from these anticipated in forward-looking statements will be discovered beneath the caption “Threat Elements” within the Firm’s Annual Report on Kind 10-Okay for the 12 months ended March 31, 2024, and within the Firm’s different filings with the Securities and Trade Fee, together with its quarterly Studies on Kind 10-Q. All forward-looking statements contained on this press launch communicate solely as of the date on which they have been made. Besides as could also be required by legislation, the Firm doesn’t intend, nor does it undertake any obligation, to replace this data to replicate future occasions or circumstances.
Firm Contact:
Jim Frakes
Chief Government Officer and Chief Monetary Officer
Aethlon Medical, Inc.
Jfrakes@aethlonmedical.com
Investor Contact:
Susan Noonan
S.A. Noonan Communications, LLC
susan@sanoonan.com



SOURCE Aethlon Medical, Inc.
Disclaimer/Disclosure: Athelon Medical, Inc. (AEMD) is a paid featured medical tech inventory on Investor concepts Extra disclosure: Investorideas.com is a digital writer of third celebration sourced information, articles and fairness analysis in addition to creates authentic content material, together with video, interviews and articles. Authentic content material created by investorideas is protected by copyright legal guidelines aside from syndication rights. Our web site doesn’t make suggestions for purchases or sale of shares, providers or merchandise. Nothing on our websites needs to be construed as a suggestion or solicitation to purchase or promote merchandise or securities. All investing includes danger and potential losses. This web site is at present compensated for information publication and distribution, social media and advertising and marketing, content material creation and extra. Extra disclosure: Contact administration and IR of every firm immediately relating to particular questions.


